
The value propositions and business model include:
Obesity, Diabetes & Metabolic Syndrome are the fastest growing health markets in the world and all can lead to MASH
MASH affects up to 20 million people in the U.S., and is the leading indication for liver transplant…
75 million of adults in the U.S. have MASLD
>25% of US adults have MASLD, with 20% progressing to MASH
Source: Int J Mol Sci, 2019; www.liverfoundation.org; Clin Diabetes and Endocrinology, 2020
Worldwide prevalence of MASLD is considerably higher than previously estimated and is continuing to increase at an alarming rate. The overall prevalence of MASLD worldwide is estimated to be 32.4%
(95% CI 29.9–34.9).- Lancet, Gastroenterology & Hepatology, Sept 2022
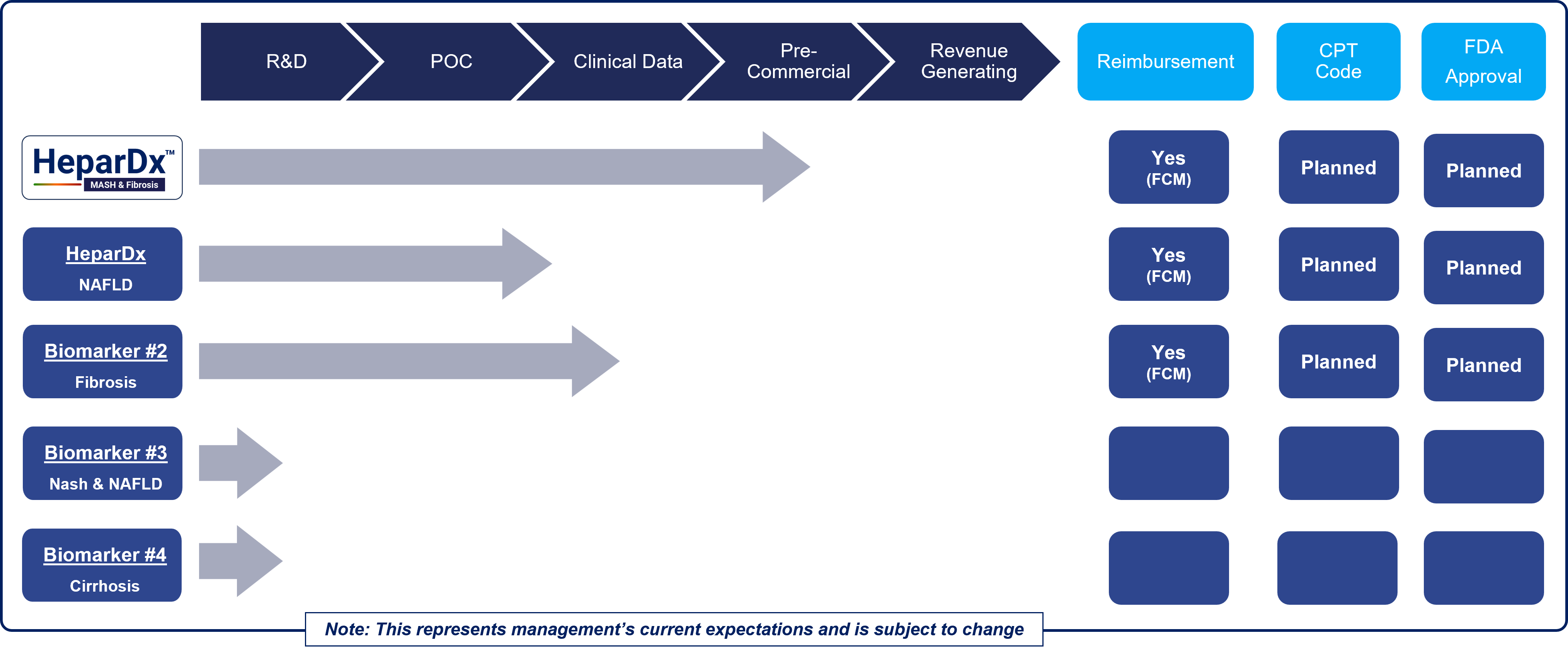
Pipeline and Milestones for Next Generation NITs in MASLD, MASH, Liver Fibrosis, and Liver Cirrhosis
Other Tests: Key Facts
