
Simple Blood-Based Diagnosis of MASH
and Liver Fibrosis
The value propositions and business model include:
- Provide an alternate / complement to liver biopsies to change clinical practice and industry guidelines
- Enable PCPs and GPs to identify MASH patients before significant disease progression can occur
- Monitor patient response to new medications
- Lower costs to the Healthcare system
- Three sources of future revenue generation:
- Commercialization of an accurate, blood-based diagnostic test to detect MASH
- Partnership(s) with CROs to reduce clinical trial screen failure rates
- Development of companion diagnostics
MASH: The Unmet Need
Obesity, Diabetes & Metabolic Syndrome are the fastest growing health markets in the world and all can lead to MASH
20 Million
MASH affects up to 20 million people in the U.S., and is the leading indication for liver transplant…
75 Million
75 million of adults in the U.S. have MASLD
25 Million
>25% of US adults have MASLD, with 20% progressing to MASH
Source: Int J Mol Sci, 2019; www.liverfoundation.org; Clin Diabetes and Endocrinology, 2020
Facts & Prevalence
- 5% of US adults with MASH
- 30-60% of T2D patients have MASLD
- 75% of overweight people have MASLD
- 35% of MASH cases progress to liver fibrosis
Worldwide prevalence of MASLD is considerably higher than previously estimated and is continuing to increase at an alarming rate. The overall prevalence of MASLD worldwide is estimated to be 32.4%
(95% CI 29.9–34.9).- Lancet, Gastroenterology & Hepatology, Sept 2022
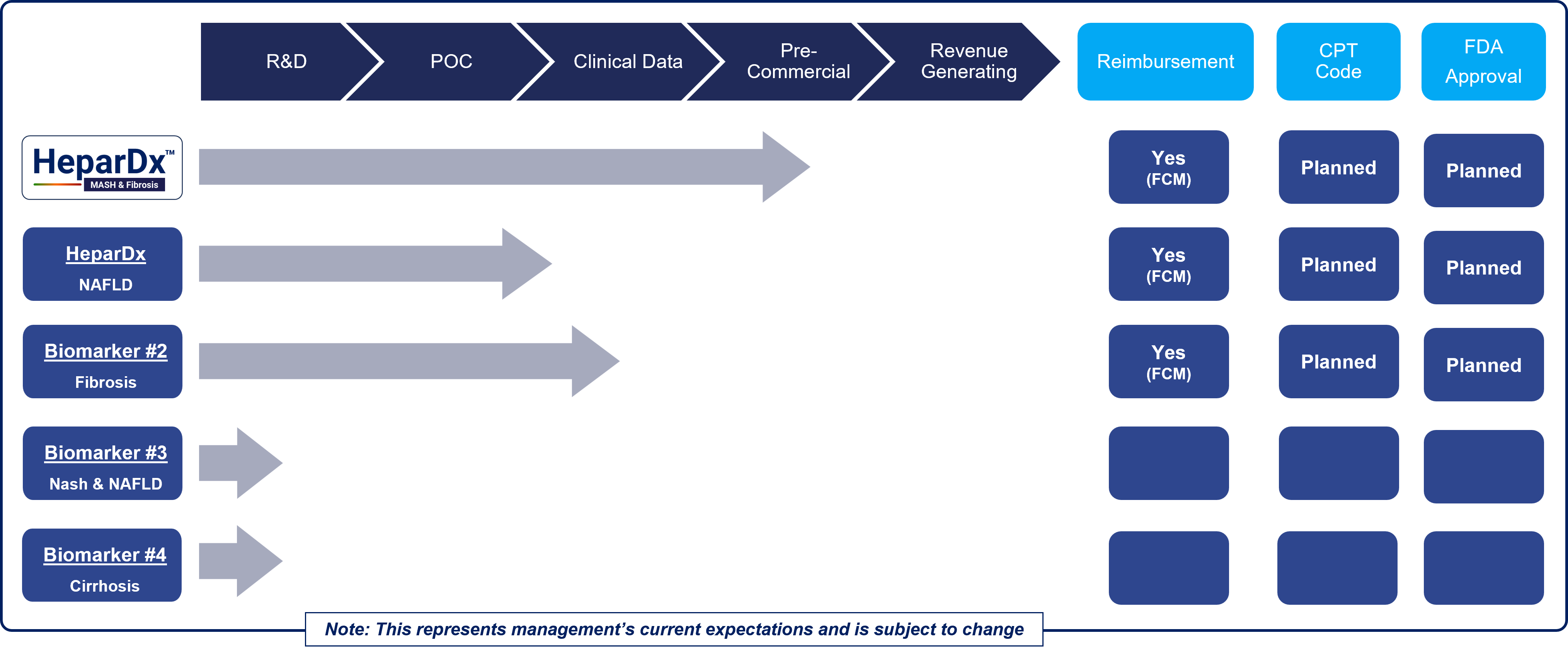
Pipeline and Milestones for Next Generation NITs in MASLD, MASH, Liver Fibrosis, and Liver Cirrhosis
Other Tests: Key Facts
- Liquid biopsy tests are gaining traction in the diagnostics industry as a viable alternative to traditional invasive procedures
- Three non-specific diagnostic tests have been approved by the FDA: Siemens’ ELF Test (a 3 biomarker blood assay), EchoSens’ FibroScan (an ultrasound device), and Perspectum Diagnostics’ LiverMultiScan (an injectable imaging marker)
- Other available NITs include: NIS4, OWLiver, FibroSURE, FIB-4, AGILE3+, LIVERFASt, and many others, but in large scale, multi-year studies such as LITMUS and NIMBLE, no biomarker has shown >0.9 AUROC.


